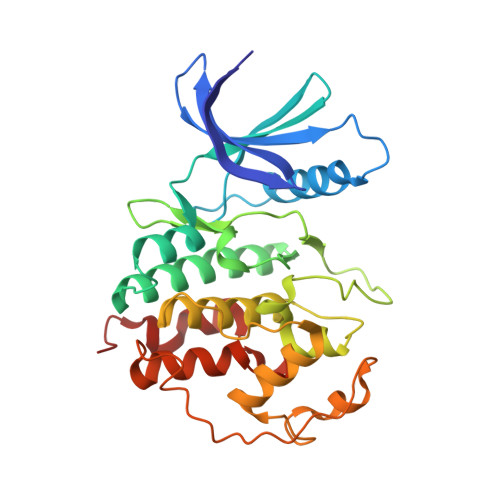
Discovery and Optimization of Highly Selective Inhibitors of CDK5.
Daniels, M.H., Malojcic, G., Clugston, S.L., Williams, B., Coeffet-Le Gal, M., Pan-Zhou, X.R., Venkatachalan, S., Harmange, J.C., Ledeboer, M.(2022) J Med Chem 65: 3575-3596
- PubMed: 35143203
- DOI: https://doi.org/10.1021/acs.jmedchem.1c02069
- Primary Citation of Related Structures:
7VDP, 7VDQ, 7VDR, 7VDS, 7VDU - PubMed Abstract:
Autosomal dominant polycystic kidney disease (ADPKD) is the most prevalent monogenic human disease, but to date, only one therapy (tolvaptan) is approved to treat kidney cysts in ADPKD patients. Cyclin-dependent kinase 5 (CDK5), an atypical member of the cyclin-dependent kinase family, has been implicated as a target for treating ADPKD. However, no compounds have been disclosed to date that selectively inhibit CDK5 while sparing the broader CDK family members. Herein, we report the discovery of CDK5 inhibitors, including GFB-12811 , that are highly selective over the other tested kinases. In cellular assays, our compounds demonstrate CDK5 target engagement while avoiding anti-proliferative effects associated with inhibiting other CDKs. In addition, we show that the compounds in this series exhibit promising in vivo PK profiles, enabling their use as tool compounds for interrogating the role of CDK5 in ADPKD and other diseases.
Organizational Affiliation:
Goldfinch Bio, 215 First Street, Cambridge, Massachusetts 02142, United States.